Pfizer Discontinues Development of Weight Loss Drug: How Its Stock Reacted

5 minutes for reading
Pfizer Inc. (NYSE: PFE) announced on 26 June 2023 that it is discontinuing further development of lotiglipron, a weight loss drug. In this article, we will talk about why this decision was made, whether the pharmaceutical corporation will remain in the weight loss market, and how the stock reacted to the news.
Pfizer in brief
Pfizer Inc. is a US pharmaceutical company involved in the manufacture and distribution of a wide range of medications and consumer health products. It produces Lipitor, Viagra, and Comirnaty, which are among the best-selling medications in the world. Pfizer is present in over 100 countries and holds leading positions in the markets of the US, Europe, Japan, and China. Its revenue exceeded 100 billion USD in 2022, with the US market share accounting for nearly 42%.
Why did Pfizer discontinue the development of lotiglipron?
Lotiglipron is an experimental drug that was developed by Pfizer Inc. for the treatment of obesity and Type 2 diabetes mellitus. Unlike most competing drugs that require injection, this one was formulated as a tablet.
The development of lotiglipron began in 2018, and one year later, the first phase of trials showed that the drug was well tolerated and had favourable pharmacokinetic properties. The second phase of research, involving 1,200 participants, started in 2020. It revealed that some patients taking lotiglipron had elevated liver enzymes, indicating potential liver damage. On 26 June 2023, the company decided to terminate the development of this medication.
How did investors react to the news?
Investors learnt about the discontinuation of lotiglipron’s development on 26 June before the start of the trading session. Prior to the market opening, Pfizer Inc. stock dropped from 38.30 to 37.00 USD per share and tumbled to 36.15 USD within the first hour of the trading session, resulting in a loss of 5.62%.
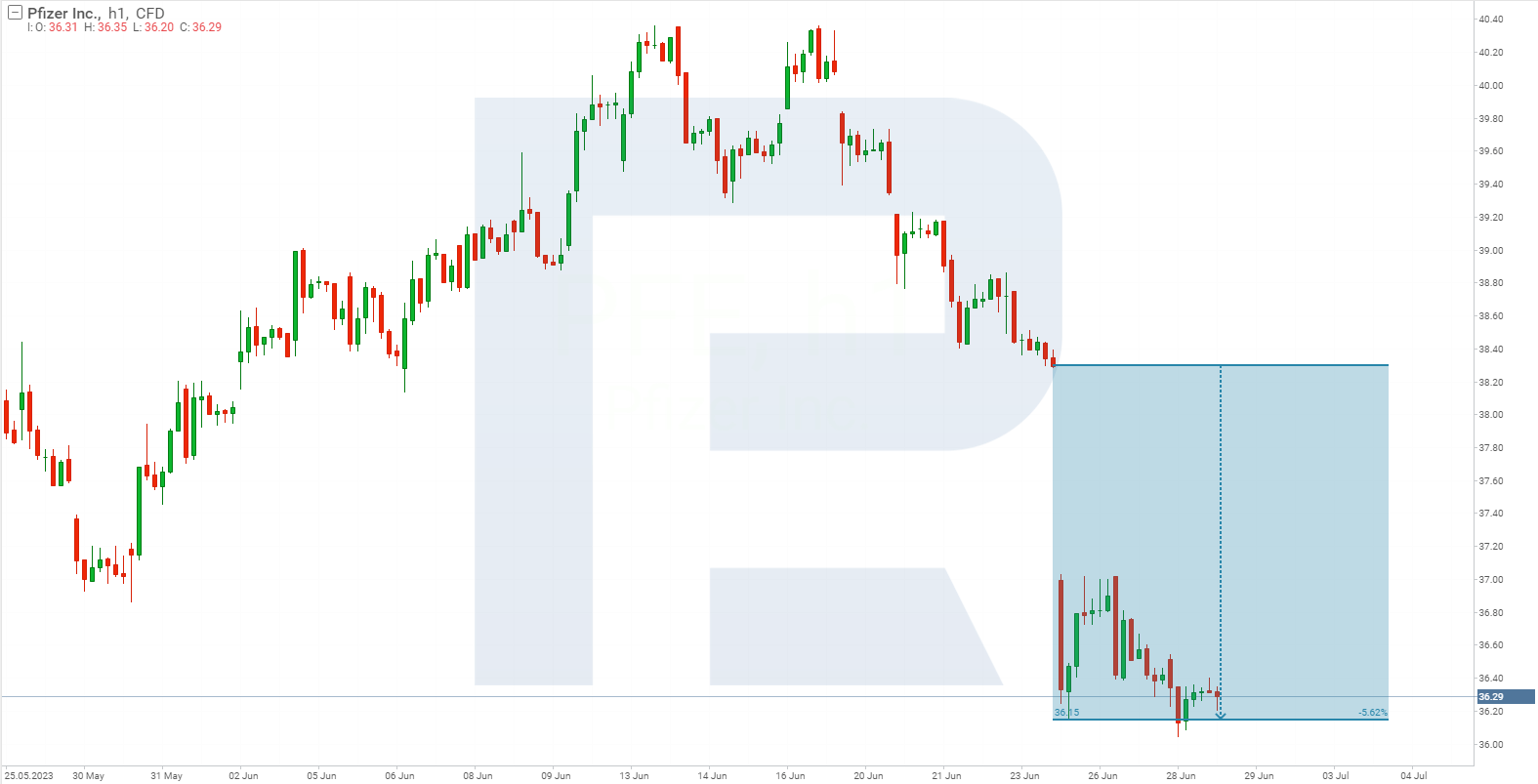
A week had passed since the news was published, but Pfizer Inc. stock had not recovered from the decline. According to the Centers for Disease Control and Prevention (CDC), from 2017 to March 2020, 41.9% of American adults exhibited signs of obesity. Considering this statistic, and the fact that the US market accounts for approximately 42% of the pharmaceutical corporation’s annual revenue, it can be assumed that investors’ bet on lotiglipron and their reaction to the news was predictable.
Will Pfizer exit the weight loss drug market?
Pfizer Inc.’s exit from this segment would positively impact the business of Novo Nordisk A/S (NYSE: NVO) and Eli Lilly and Company (NYSE: LLY), which are developing similar medicines. However, since 2018, the company has been working not only on lotiglipron, but also on danuglipron. This is another drug for the treatment of obesity and Type 2 diabetes, which differs from lotiglipron in terms of lower efficacy and administration method. The corporation expects the second phase of danuglipron’s research to be completed by the end of 2023.
Which Pfizer medications are currently in the registration stage?
Oncology diseases:
- Elranatamab (treatment of blood cancer)
- Braftovi (treatment of various types of cancer)
- Talzenna (treatment of breast cancer)
Inflammatory processes and immunology:
- Ritlecitinib (treatment of hair loss)
- Etrasimod (treatment of immune disorders)
Rare diseases:
- Somatrogon (growth hormone)
Vaccines:
- Comirnaty (treatment of COVID-19)
- BNT162b2 bivalent (treatment of COVID-19)
- PF-06928316 (treatment of respiratory tract infections)
- PF-06886992 (treatment of meningococcal infections)
Which of the above medications have been approved by the FDA?
On 23 June 2023, the Food and Drug Administration (FDA) approved Ritlecitinib (Litfulo), an oral medicine for the treatment of severe alopecia in adults and teenagers aged 12 and above. This is the first and only medication to receive regulatory approval for treating children with this condition. Clinical trials showed that Ritlecitinib demonstrated a high level of efficacy and safety in patients with alopecia areata, which is the most common form of hair loss.
According to Insight Partners, the market volume for hair loss prevention medications reached a value of 23.5 billion USD in 2021. It is expected to reach 31.5 billion USD by 2028, showing an average annual growth of 4.2%.
Summary
In late June, it was announced that Pfizer Inc. is discontinuing further development of lotiglipron, a weight loss medication. This news led to a marked decrease in the company’s stock value. However, the corporation continues to work on another weight loss drug (danuglipron) and seeks to strengthen its position in the weight loss drug market.
At the time of writing this article, Pfizer Inc. had over 100 medications in the development stage, with more than 10 drugs undergoing the registration process, and one of them had already received FDA approval. Pfizer Inc.’s revenue exceeded 100 billion USD in 2022, which suggests that the company has created favourable conditions for increased funding of new research. Positive results of these efforts can have a substantial impact on the corporation’s financial position and the value of its stock in the future.
* – Past performance is not a reliable indicator of future results or future performance.
The material presented and the information contained herein is for information purposes only and in no way should be considered as the provision of investment advice for the purposes of Investment Firms Law 87(I)/2017 of the Republic of Cyprus or any other form of personal advice or recommendation, which relates to certain types of transactions with certain types of financial instruments.















 are complex instruments and come with a high
are complex instruments and come with a high  of losing
of losing  rapidly due to
rapidly due to  . 65.68% of retail investor accounts lose
. 65.68% of retail investor accounts lose  when trading
when trading  with this provider. You should consider whether you understand how CFDs work and whether you can afford to take the high
with this provider. You should consider whether you understand how CFDs work and whether you can afford to take the high